Chlorine Dioxide is The Gold Standard of Complete Water Disinfection Solutions
Chlorine dioxide (ClO2) is a yellow-green gas with an odor similar to chlorine with excellent distribution, penetration, and sterilization abilities due to its gaseous nature. . Molecular weight of 67.45, Melting point of -59 degree C, Boiling point 11oC . Chlorine dioxide has been recognized as a disinfectant since the early 1900s and has been approved by the US Environmental Protection Agency (EPA) and the US Food and Drug Administration (FDA) for many applications. It has been demonstrated effective as a broad spectrum, anti-inflammatory, bactericidal, fungicidal, and virucidal agent, as well as a deodorizer, and also able to inactivate beta-lactams and destroy both pinworms and their eggs. In pure form Chlorine dioxide is a hazardous gas as most people are not likely to breath air containing dangerous level of Chlorine dioxide as it rapidly breaks down in air as Chlorine gas and Oxygen. Chlorine dioxide is always made at the location where it is used.
The US EPA regulates the maximum concentration of Chlorine dioxide in drinking water to be no greater than 0.8 parts per million (ppm).
The US Occupational Safety and Hazard Administration (OSHA) has set Permissible Exposure Limit (PEL) for Chlorine dioxide is 0.1 per parts per million (ppm) or 0.3 milligrams (mg) per cubic meter (m3) at work place.
Chlorine dioxide is not a cure or treatment for medical ailments.
Molecular Size Matters-Smaller Than any Virus
The size of a chlorine dioxide gas molecule is 0.124 nm, much smaller than microorganisms and viruses, allowing the gas to easily penetrate into any areas where these microorganisms might be concealed.
Mechanism
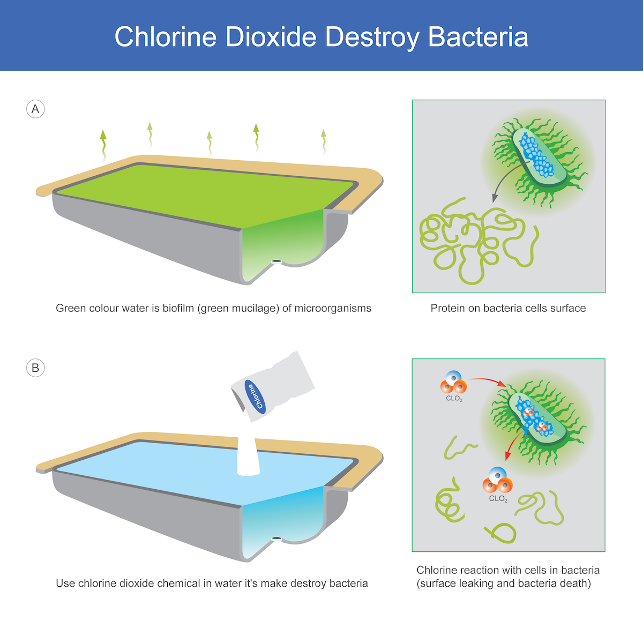
Chlorine dioxide acts as an oxidizing agent. By taking electrons from microbe's bond, the microbe's molecular bond weakens and the cell breaks apart. Thus the process alters the proteins involved in their structure and decreases enzyme function, thus resulting in the death of the microbes,.This attacks on many protein simultaneously prevents the microorganism from mutating and developing resistance.
ClO2 Timeline
1811 first discovered by Sir Humphrey Davey. 1944 First commercial application. Used as a biocide/taste and odour control agent in domestic water at Niagara Falls in the USA. 1977 Three thousand municipal water systems achieving biological control using Chlorine Dioxide.1980’s Chlorine Dioxide gradually replacing Chlorine in many industries. Pulp and Paper industry - used as a bleaching agent. Industrial water treatment - used as a biocide and as an odour control agent. Food processing - used as a sanitiser.1990’s Increasingly used for the secondary disinfection of potable water. 2005 – New generation equipment and control technology introduces ClO2 as a practical alternative to many industrial applications. 2018- FES scientists developed cost-efficient way to produce Chlorine dioxide with minimum Chemical input.

Advantages over Other Oxidizing Biocides
Chlorine dioxide is superior to other similar oxidizing biocides like Ozone, Hydrogen peroxide and Peracetic acid in terms of stability, difficulty in handling and applying safely and yet more powerful disinfectant.
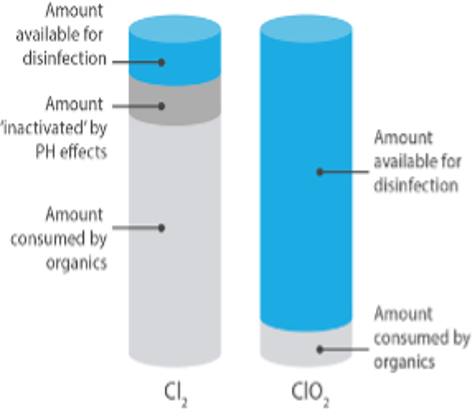
Chlorine Dioxide has a number of advantages over other oxidizing biocides that make it more suitable for use in a number of water treatment applications. Chlorine Dioxide as an oxidizing biocide has a significantly lower oxidation strength – this means that it reacts with fewer compounds yet is strong enough to attack the disulphide bonds found in the membranes of bacteria and other biological material.
The selective oxidation allows the Chlorine Dioxide biocide to be targeted where it is needed most, disinfecting areas quickly and at lower dose rates. That leads to greater cost efficiencies. As an example, Hydrogen Peroxide in water treatment, dosing rates 10-30 times greater than ClO2.
Advantages Over Chlorine
Greater cost efficiencies & Yield - Chlorine Dioxide has a higher oxidation capacity, and a lower oxidation strength thus making it at least 2.6 times more powerful per ppm according to WHO CT values.
Work over wide pH range: Unlike Chlorine, Chlorine dioxide is not pH dependent, and it remains in gaseous state in water. Chlorine is almost ineffective above pH8
Zero carcinogenic by-products & no bad taste formation in water : Unlike Chlorine, Chlorine Dioxide acts only by oxidation and does not combine with organic compounds to form environmentally hazardous by-products such as Trihalomethane and other chlorinated organic compounds that have been listed as potentially carcinogenic.
Less corrosive: Being lower oxidation potential, Chlorine Dioxide does not hydrolyze to form an acid, and therefore is less corrosive.
Effective against complex organisms - Chlorine Dioxide has been found to be effective against complex organisms such as cysts and protozoa including Cryptosporidium, Giardia, and amoeba. Chlorine is not.
Destroys biofilm completely at source: Biofilms affect the safety of water distribution system and heat exchange in cooling tower. By penetrating polysaccharide layers, ClO2 destroys pseudomonas and other base bacteria within the biofilm.
,
Broad-spectrum Powerful Disinfection Power
Chlorine dioxide (ClO2) is a highly effective, environmentally-friendly biocide. It is a stable, dissolved gas that is a strong bactericide and virucide at concentrations as low as 0.1 ppm at a minimum contact time. A selective oxidant that attacks planktonic and sessile bacteria, disinfects surfaces, and rapidly reduces biofilms. it is highly effective against pathogenic organisms such as Legionella, amoebal cysts, Giardia cysts, E. Coli, MRSA, ABR, AGR bacteria and Cryptosporidium. ClO2 reduces biofilms so bacterial regrowth is significantly impeded.
ClO2 does not ionize to form weak acids (as chlorine and bromine do) in aqueous solutions. This allows
ClO2 to be effective over a wide pH range.
ClO2 is shown to be an effective disinfectant at residual concentrations between 0.2 and 0.8 ppm. ClO2 penetrates the cell wall of the microorganism and disrupts metabolic functions. This is more efficient than other oxidizers that “burn” whatever they come in contact with. This allows lower effective concentrations to be used. ClO2, like ozone, is a dissolved gas that penetrates biofilm by molecular diffusion.
Aldehydes
Chlorine Dioxide oxidizes formaldehyde to formic acid and finally to carbon dioxide. Para formaldehyde can be depolymerized and eliminated completely by oxidation with Chlorine Dioxide.
Amines and Mercaptans
The major sources of odorous substances such as mercaptans and substituted amines include the chemical and petroleum industries, cooking and sanitary processes, animal feedlots and rendering plants.
Chlorine dioxide destroys the mercaptan odor. Similarly, chlorine dioxide reacts with organic sulphides and disulphides destroying the original odor. Secondary and tertiary amines are also present in many wastewaters, causing their own unique odor problems.
THM Precursors
Chlorine Dioxide reacts differently than Chlorine with the precursors of Tri-halomethane (THM) such as Humic and Fulvic acids. That is why Chlorine dioxide don’t form TTHMs by-products. Chlorine reacts oxidation and electrophilic substitution to yield both volatile and non-volatile chlorinated organic substances (THMs). However, Chlorine dioxide primarily by oxidation to make them non-reactive or unavailable for THM production.
Pesticides
Chlorine Dioxide can oxidize toxic materials to fewer toxic materials. Specifically, Methylchlor (DMDT) , parathion and Adrian react with ClO2 including such as paraquat and diquat are eliminated within a few minutes.
Algae/Slime
Chlorine Dioxide has been effective in controlling algae by altering the chlorophyll metabolism, they are destroyed. The reaction of Chlorine Dioxide with algae and their essential oils forms tasteless, odorless substances. The treatment is done by night to prevent photolytic decomposition of ClO2. A dosage of 1mg/ litre.
Sulphides
Many industrial processes produce sulphide-containing gases and waste products. 5.2 parts by weight of Chlorine Dioxide instantaneously oxidizes 1 part by weight of hydrogen sulphide.
Nitrogen Compounds
Chlorine Dioxide has been used to scrub NO and NO2 contaminants. This process is particularly convenient for continuous operation.
Cyanides
Chlorine Dioxide oxidizes simple cyanide to cyanate (a less toxic substance) and/or carbon dioxide and nitrogen. Cyanide compounds originate from processes such as metal plating, steel case hardening, pickle liquor neutralizing, gold and silver ore refining and blast furnace stack gas scrubbing.
Iron & Manganese
Chlorine dioxide reacts rapidly with soluble forms of iron and manganese to form precipitates that can be removed through sedimentation and filtration.
Odor Neutralizer
Chlorine dioxide is a potent odor eliminating gas with strong oxidizing properties,
Biocidal Efficacy as per US EPA
Chlorine dioxide is known as the most effective biocide available worldwide. Chlorine dioxide is arguably the best biocide approved by the USEPA and FDA. Unlike chlorine, it does not form
carcinogenic disinfectant byproducts (THMs and HAAs), and it is more effective at lower dosing levels.
Chlorine dioxide meets all disinfection criteria approved by US EPA.
1. a 99.9% reduction in Giardia lamblia (3 log reduction)
2. zero lactose fermenting coliform
3. less than 10 cfu/mL non-lactose fermenting coliforms and
4. 99.99% reduction in enteric virus (4 log) concentrations.
Regulatory Approvals
• United States Environmental Protection Agency (USEPA)
• United States Food and Drug Administration (FDA)
• Building Services Research and Information Association of the UK (BSRIA)
Applications
Cooling Towers and Loops: ClO2 controls algae, planktonic bacteria, biofilm and scale for maximum efficiency
of heat exchangers and ancillary equipment. ClO2 is more stable than other oxidizing biocides and
compatible with most water treatment chemistry. This "selective oxidation" makes ClO2 ideal for systems
with corrosion problems.
Cooling Towers and Loops: ClO2 controls algae, planktonic bacteria, biofilm and scale for maximum efficiency
of heat exchangers and ancillary equipment. ClO2 is more stable than other oxidizing biocides and
compatible with most water treatment chemistry. This "selective oxidation" makes ClO2 ideal for systems
with corrosion problems.
Potable Water: ClO2 is EPA-approved for both pretreatment and final disinfection of drinking water. In
pre-treatment, it effectively removes iron and manganese and promotes flocculation. It also removes
taste and odor components as well as halogenated disinfectant byproduct precursors e.g. trihalomethanes
(THM's). In post-treatment, it provides a lasting residual throughout the distribution system.
Food and Beverage: ClO2 provides excellent microbiological control in flume waters, packaging operations
and process disinfection. It is ideal for the washing of cut fruits, vegetables and poultry (FDA approved)
as well as bottling and brewery applications. ClO2 does not react with most "organics" in flume
water, which makes it a very effective disinfectant. It also neutralizes foul smelling secondary and tertiary
amines formed in the meat packing industry.
Waste Treatment and Odor Control: ClO2 safely oxidizes phenols, cyanides, aldehydes, and mercaptains,
reduced sulfur compounds and some pesticides. It is useful in both waste treatment and scrubber
systems.
